Water Electrolysis
Understanding electrocatalytic reactions in realistic environments
Motivation
Hydrogen will be critical in electrifying and decarbonizing industrial and commercial sectors. Green hydrogen from low-temperature water electrolysis is anticipated to expand its global capacity a thousand-fold by 2030. Among various electrolysis technologies, alkaline water electrolysis (AWE) faces key challenges in improving electrocatalytic reactions, particularly the oxygen evolution reaction (OER). Conducting OER in alkaline media uses first-row transition metal oxides—nearly as efficient and stable as precious metals but more abundant and cost-effective. Despite extensive research enhancing OER catalytic activity, further exploration is needed to reveal how these materials perform under varying operational conditions.
I aim to understand how electrocatalytic interfaces evolve during water-splitting reactions in realistic environments. By improving our understanding of these phenomena, we can enhance the activity and stability of electrocatalytic materials. This advancement is crucial for low-temperature water electrolysis technologies to meet industrial requirements.
Research Projects
I am particularly interested in understanding the chemical, structural, and electrochemical transformations of multi-metal electrode materials, focusing on dynamic processes at the electrode-electrolyte interface, such as corrosion, metal redeposition/incorporation, and self-healing mechanisms. In this study, our group explored the in situ incorporation of metal impurities into transition metal-based electrocatalysts. We found that metal incorporation is confined to the film’s surface, resulting in an interstratified structure that partially retains the more active, disordered phase at the surface (Figure 1). We also showed that metal incorporation can be manipulated by shifting the solubility equilibrium.
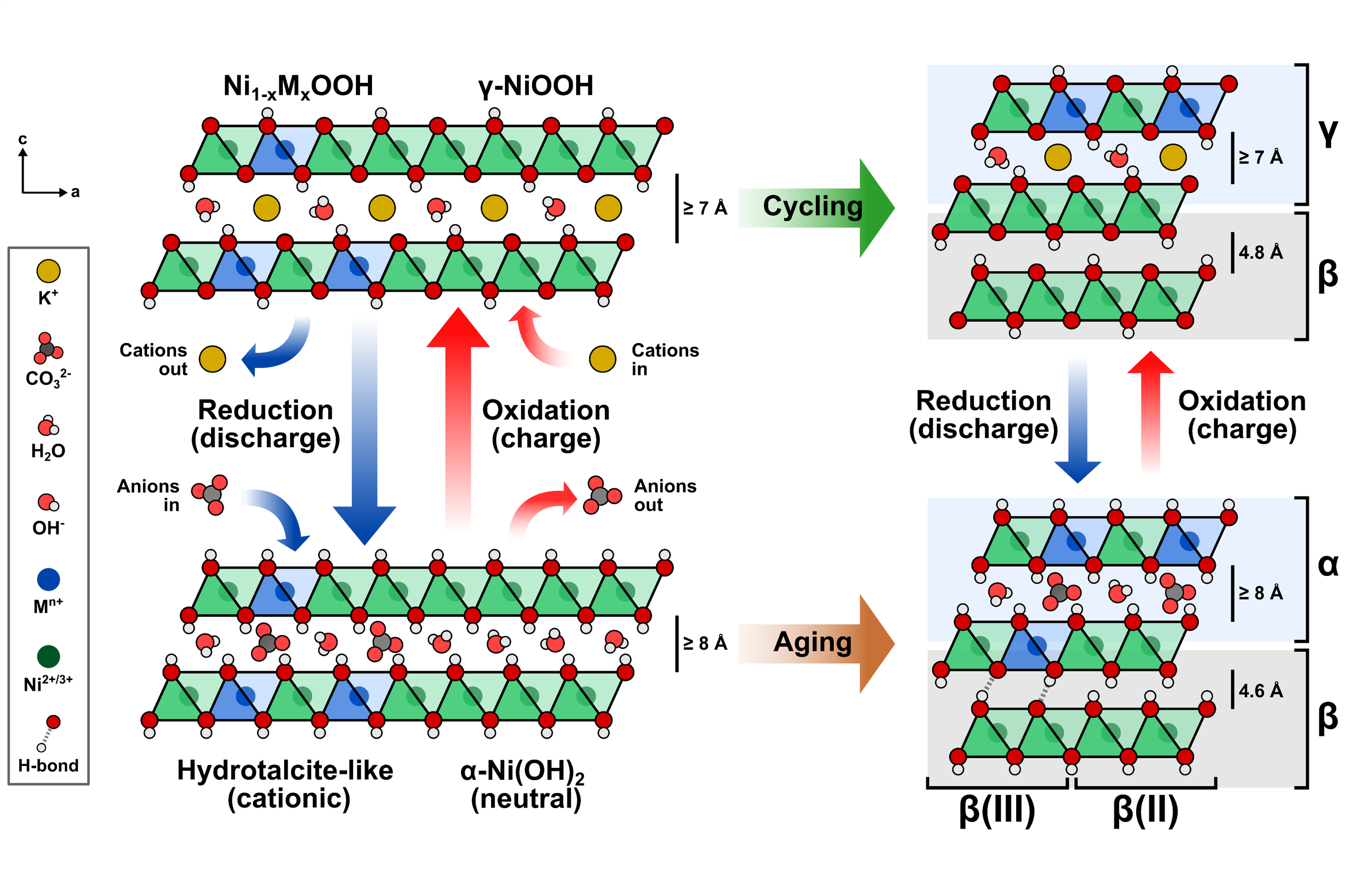
Furthermore, I am also interested in investigating the effects of intermittent water electrolysis on the surface composition and dissolution of transition metal-based electrocatalysts. By operating a lab-scale, zero-gap water electrolyzer under fluctuating currents, our group found that NiFe anodes exhibit a more stable response. In contrast, Ni anodes exhibit notable electrode potential fluctuations during electrolyzer shutdown (Figure 2).
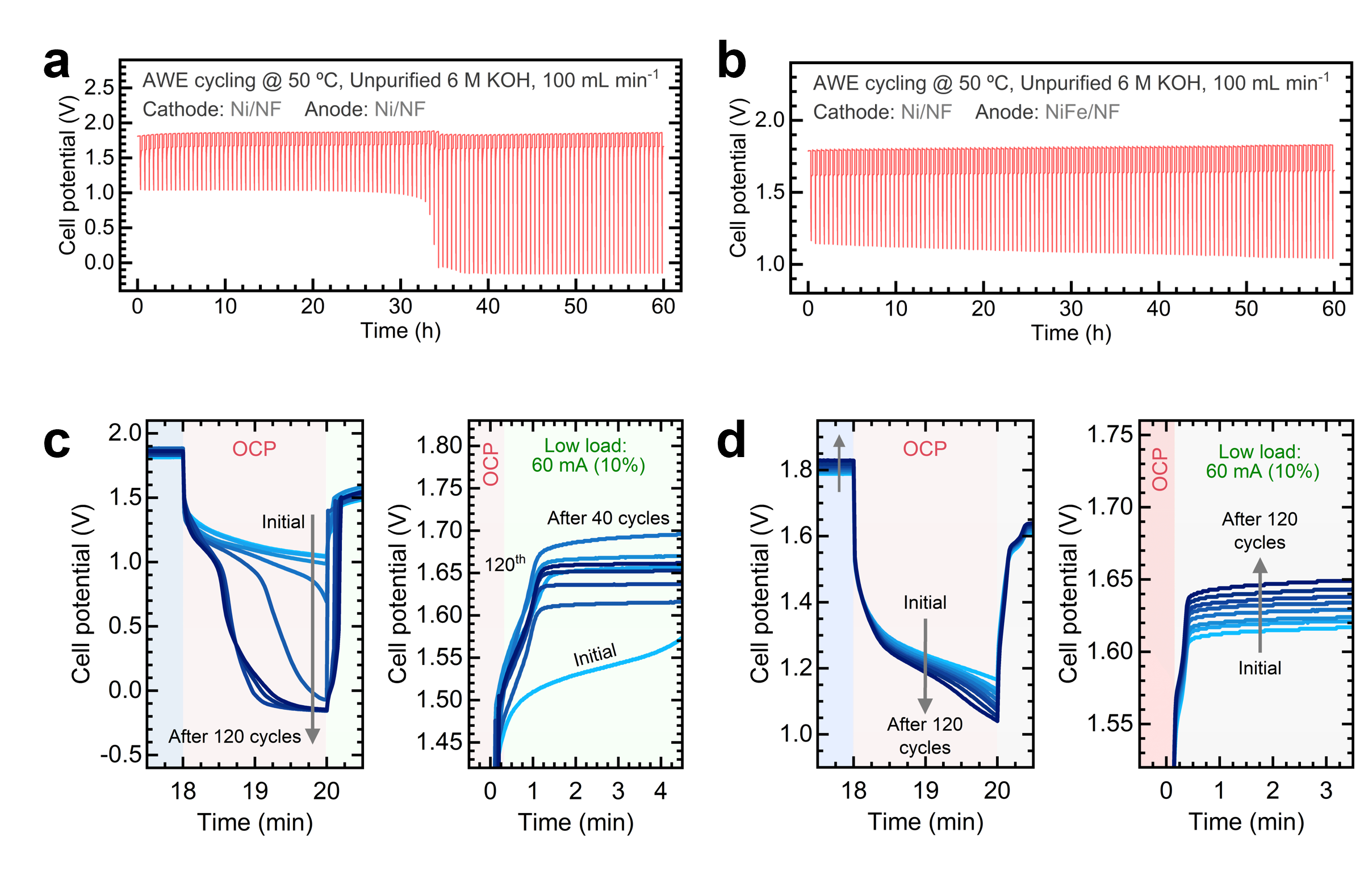
We are currently studying the effects of open circuit potential steps and reverse currents on the chemical composition of multi-metal electrocatalysts.