OER Catalysis
Studying the transformations of OER catalysts
Motivation
Electrocatalytic water splitting offers encouraging benefits for a clean and sustainable energy transition from fossil fuels. This process produces high-purity hydrogen at the cathode through the hydrogen evolution reaction (HER), while the oxygen evolution reaction (OER) occurs at the anode. The sluggish kinetics of the OER, resulting from multiple electron transfer steps and chemical intermediates, acts as a bottleneck for water splitting and dominates the overall efficiency of this technology. Electrocatalytic materials are needed to speed up the OER and make large-scale water splitting practical, abundant, efficient, and durable.
I aim to derive new catalyst compositions capable of delivering high OER activity while maintaining their long-term performance. Since these materials undergo continuous transformations during the reaction, I am particularly interested in understanding how these reconstruction processes occur and what strategies can be implemented to maximize their performance.
Research Projects
Transition metal borides, carbides, pnictides, and chalcogenides (collectively regarded as X-ides) have emerged as promising OER catalysts due to their abundance, electrical conductivity, and high catalytic activity. However, these materials demonstrate various degrees of oxidation resistance due to their chemical composition, crystal structure, and morphology differences. Thus, X-ide catalysts will experience different transformations during the strong oxidizing environment of the OER.
Our group compiled an extensive database of 900+ transition metal boride, carbide, pnictide, and chalcogenide OER electrocatalysts from over 890 peer-reviewed reports published from 2013 to 2022 (Figure 1). This effort was published as a comprehensive review in Chemical Reviews. We also designed a protocol for evaluating catalytic performance using in situ/operando characterization techniques.
In a follow-up study, we employed statistical and machine learning methods to derive catalytic performance trends from this OER catalyst database.
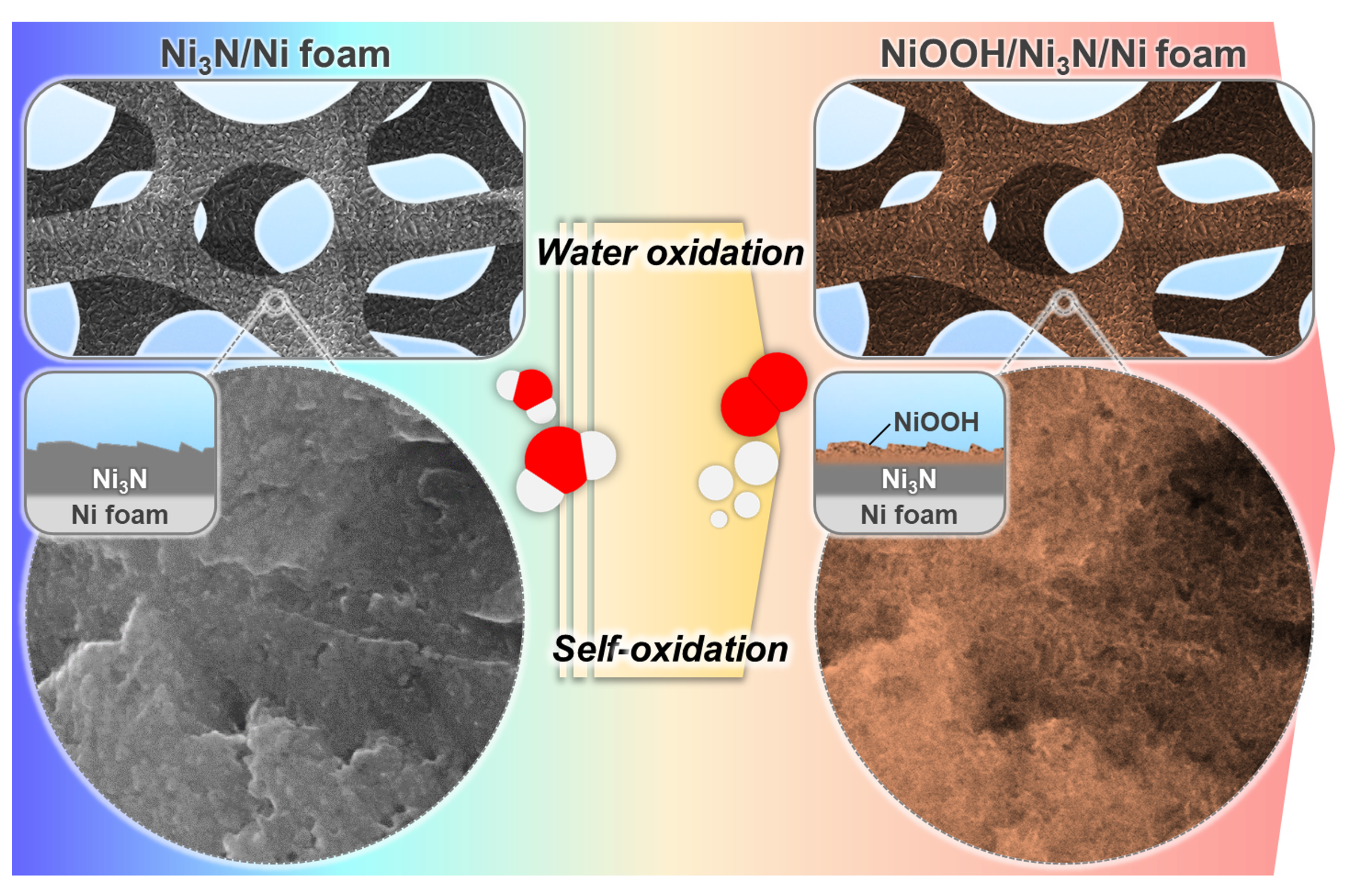
Furthermore, our group has carried out experimental research on X-ide OER catalysts. In this study, we examined the self-oxidation of nickel nitride OER catalysts and the role of Fe impurities. During OER testing, nickel nitride films were readily converted into amorphous, nano-porous NiOOH surfaces (Figure 2). A continuous increase in OER activity was observed in Fe-containing electrolytes, resulting from incorporating Fe into the NiOOH phase.
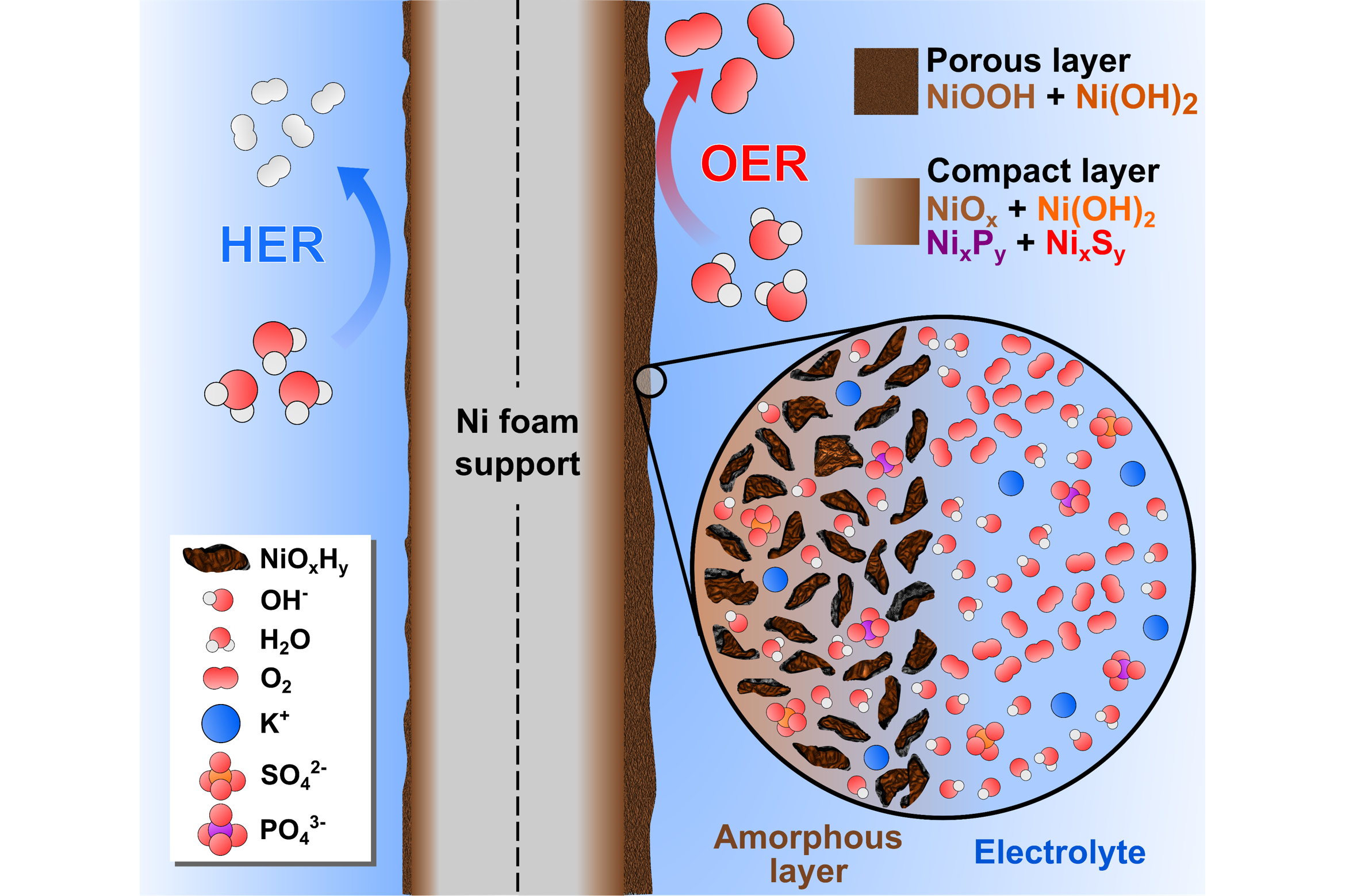
Our group also synthesized and tested nickel phosphide and sulfide water-splitting electrocatalysts using 3D-printed electrochemical flow cells in this study. From post-OER characterization, we found that the electrodeposited films underwent oxidation into amorphous NiOOH and oxidized S and P species, which dissolved into the electrolyte (Figure 3).
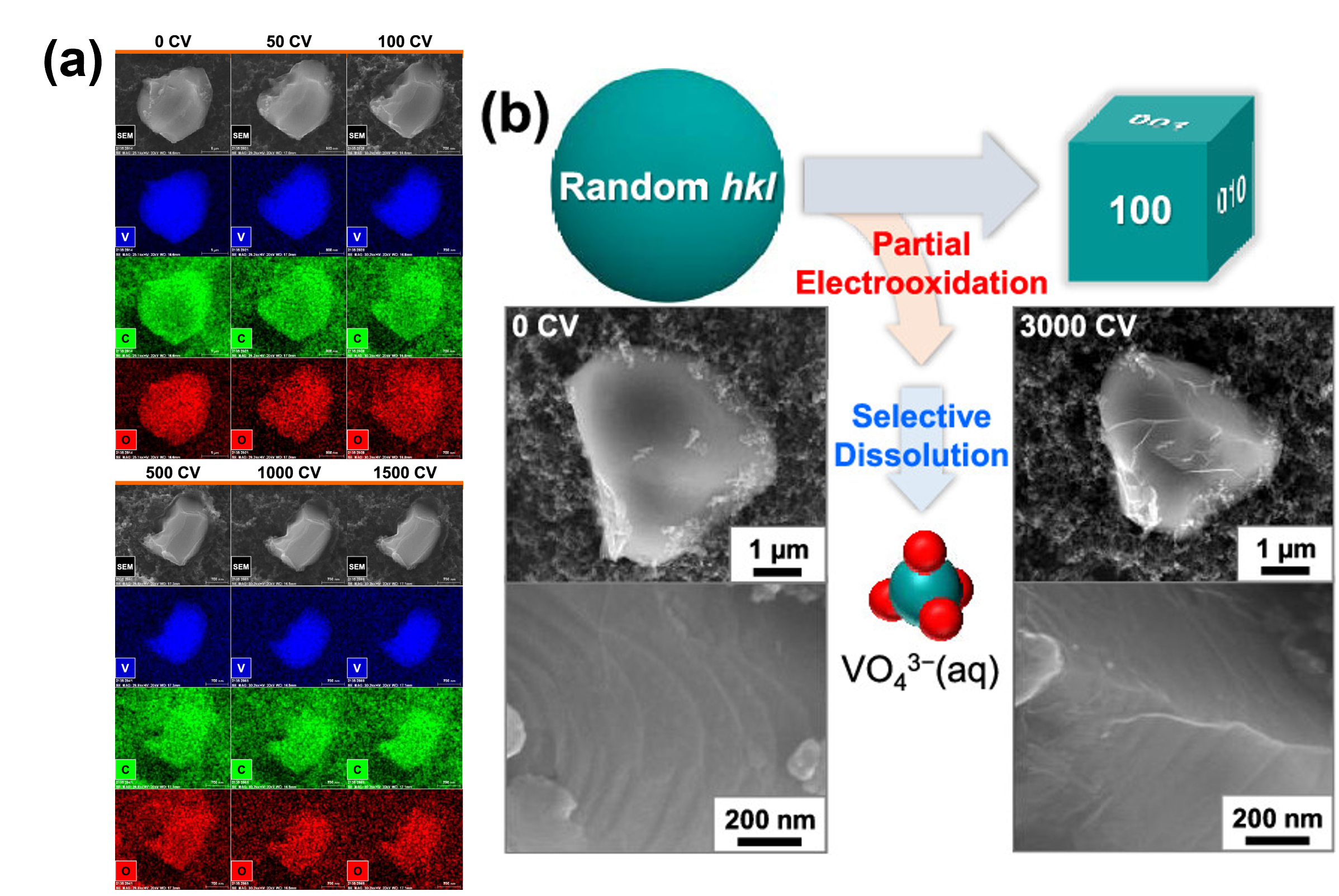
Similarly, our group found that vanadium carbide OER catalysts experience an anisotropic morphological transformation from a distorted sphere to a cuboid during the OER. In combination with DFT calculations and materials characterization, the results suggest that this morphological reconstruction occurs due to the selective dissolution of the (110) and (111) surfaces and the subsequent exposure of the relatively stable (100), (010), and (001) surfaces (Figure 4).