Practices for Electrolyte Preparation
Six practices to prepare alkaline electrolytes
Motivation
The electrolyte is a crucial component in every electrochemical system, influencing the properties of the electrode interface and performance metrics. Alkaline aqueous electrolytes, such as KOH and NaOH, are integral to various electrochemical energy devices, including alkaline electrolyzers, fuel cells, supercapacitors, and batteries. Moreover, the electrolyte influences the thermodynamics and kinetics of electrocatalytic reactions. The concentration and composition of alkaline electrolytes are critical, as impurities like metals and inaccuracies in pH measurement can lead to flawed assessments of electrochemical performance. Thus, there is a pressing need for standardized protocols to assess and enhance the quality of alkaline electrolytes to ensure accurate evaluations and comparisons of electrochemical systems.
Leveraging my background in analytical chemistry, I am dedicated to developing standardized methods and protocols in research. I am particularly focused on promoting rigor and reproducibility in electrochemistry.
Research Projects
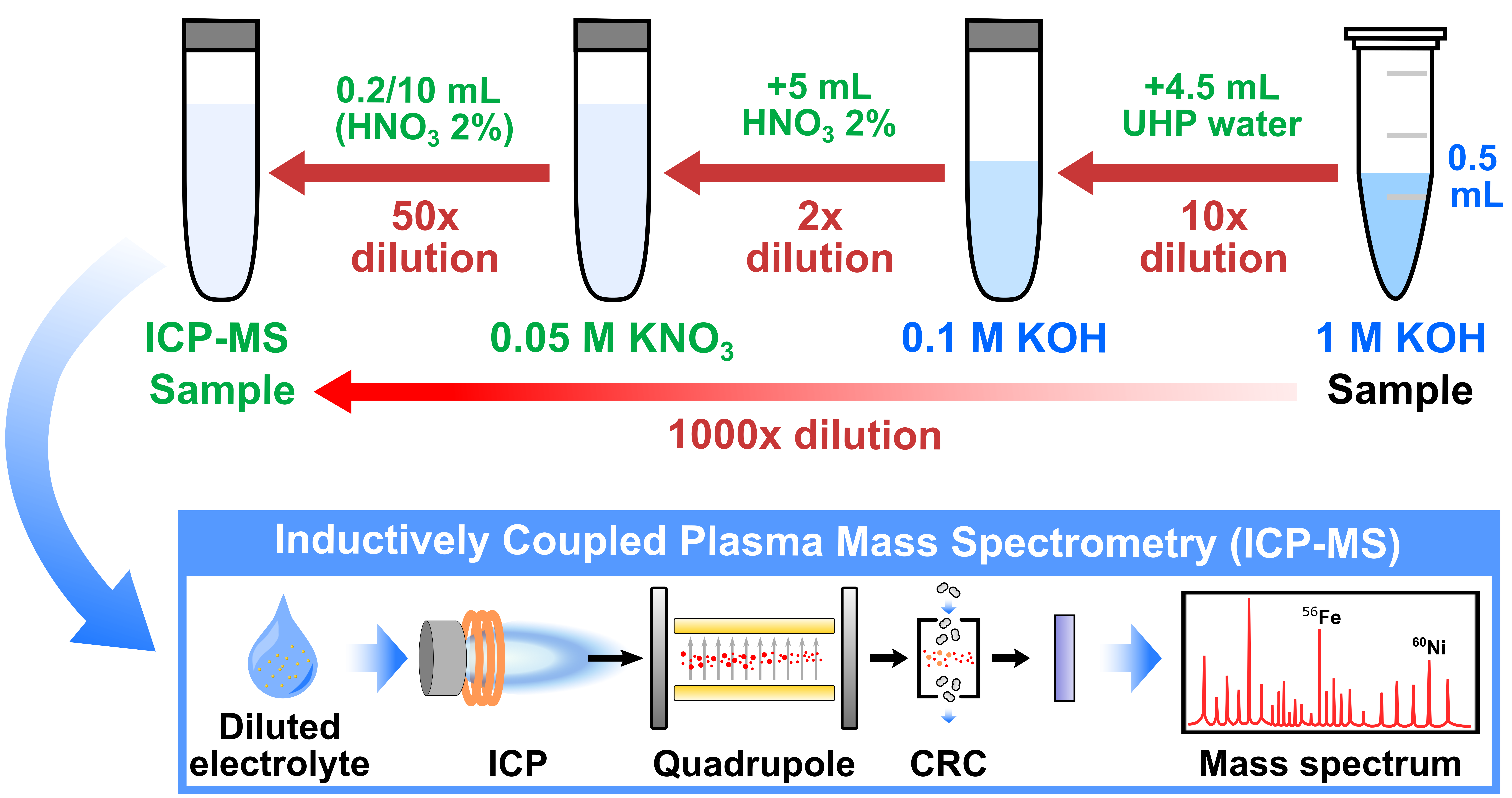
We developed a protocol to purify alkaline electrolytes (KOH, NaOH) by removing iron impurities interfering with OER catalyst testing. We outlined six practices for preparing, characterizing, and validating the quality of alkaline electrolytes and provided a comprehensive tutorial on detecting iron impurities (Figure 1).
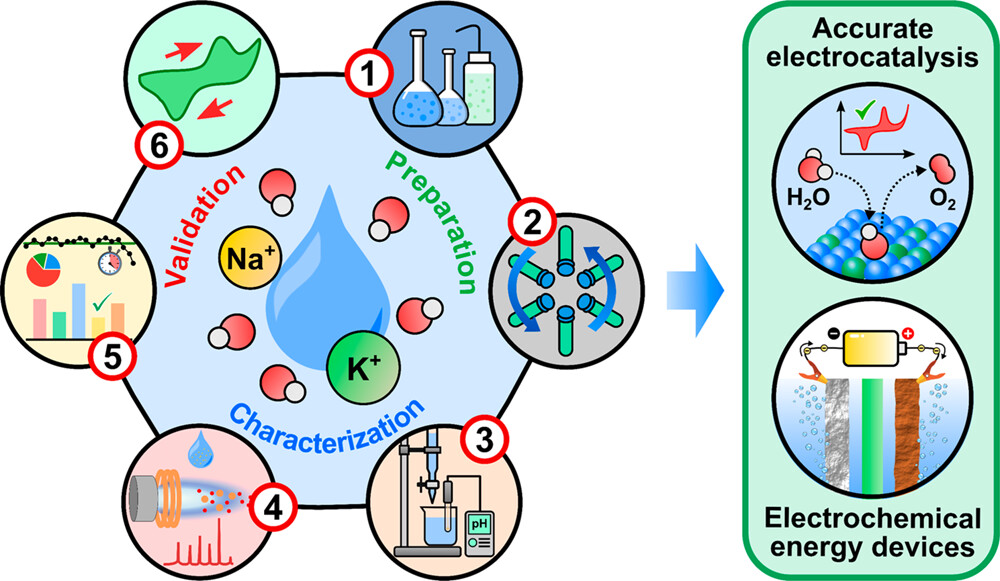
We also developed an analytical protocol (Figure 2) to determine trace metal impurities in alkaline electrolytes using inductively coupled plasma mass spectrometry (ICP-MS). This protocol is also featured in an ICP-MS course at UT-Austin.