Sustainable Flue Gas Capture
Transforming the chemical industry with sustainable electrochemistry
Motivation
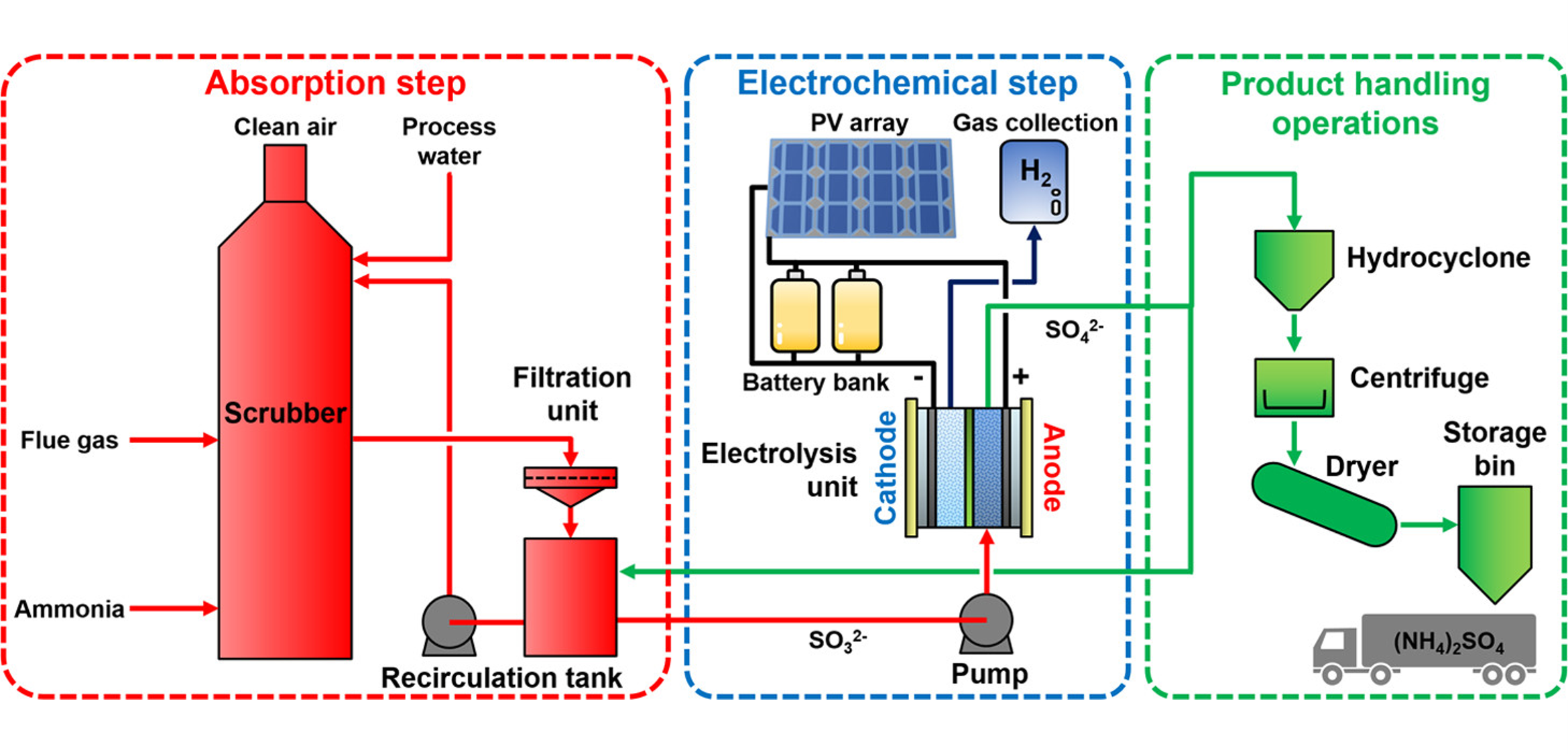
In addition to its critical role in the sulfur-based thermochemical water-splitting cycle, the electrochemical oxidation of sulfite ions offers encouraging advantages for large-scale hydrogen production and sulfur dioxide capture. Wet-type flue gas desulfurization technologies capture SO2 from power plant gas streams using aqueous ammonia as an absorbent, followed by oxidation to produce ammonium sulfate, a valuable fertilizer. When coupled with renewable energy sources like solar and wind power, electrochemical processes enable the sustainable conversion of chemical energy into electrical work. Thus, replacing conventional chemical oxidation with electrochemical steps could enable the extraction of profitable byproducts from waste in a more sustainable approach (Figure 1).
I aim to develop new strategies for the electrochemical oxidation of sulfur species and other industrially relevant chemicals, drawing on my chemical engineering expertise to address key challenges such as maximizing energy efficiency, enhancing catalytic performance, and designing and scaling electrolyzer configurations.
Research Projects
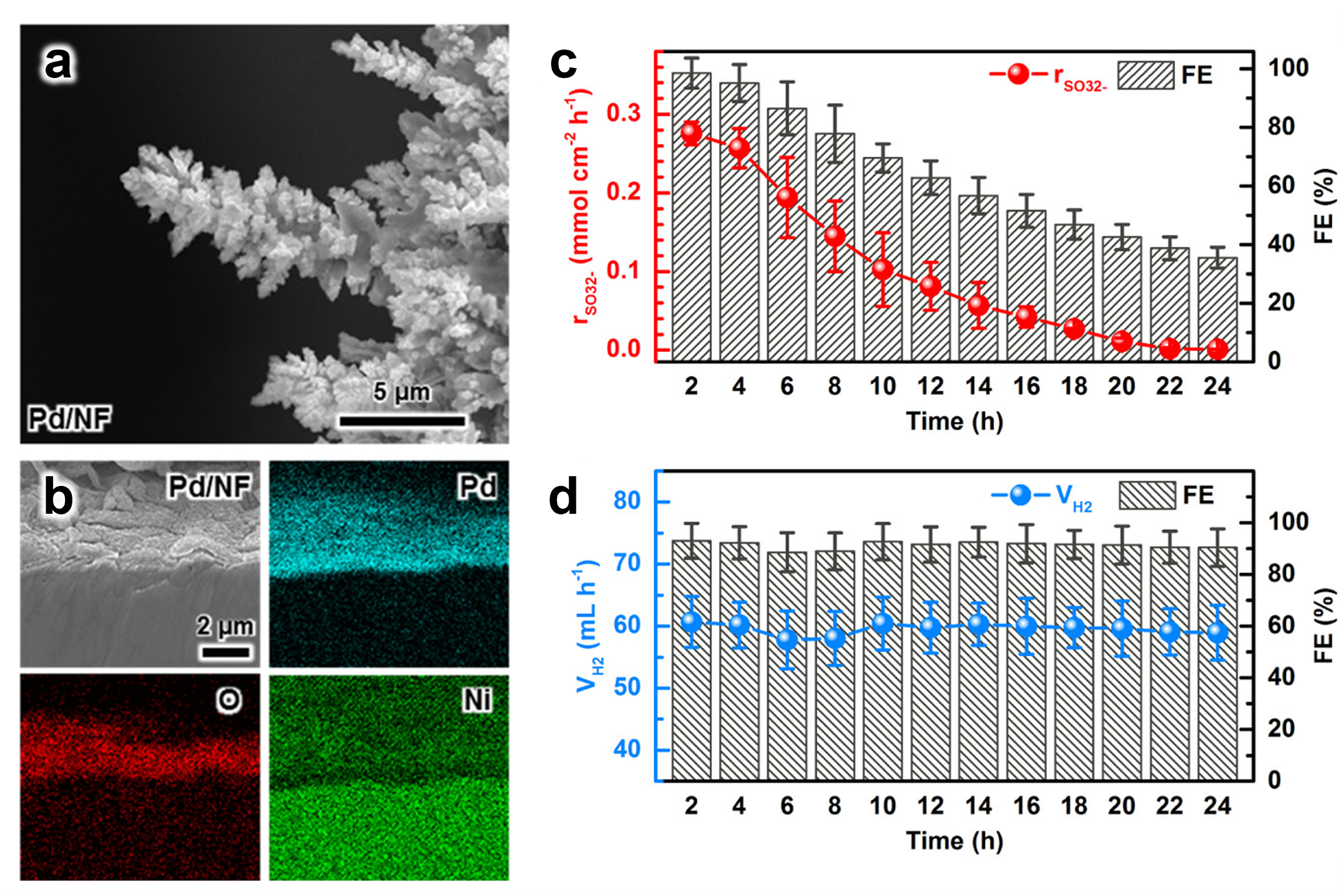
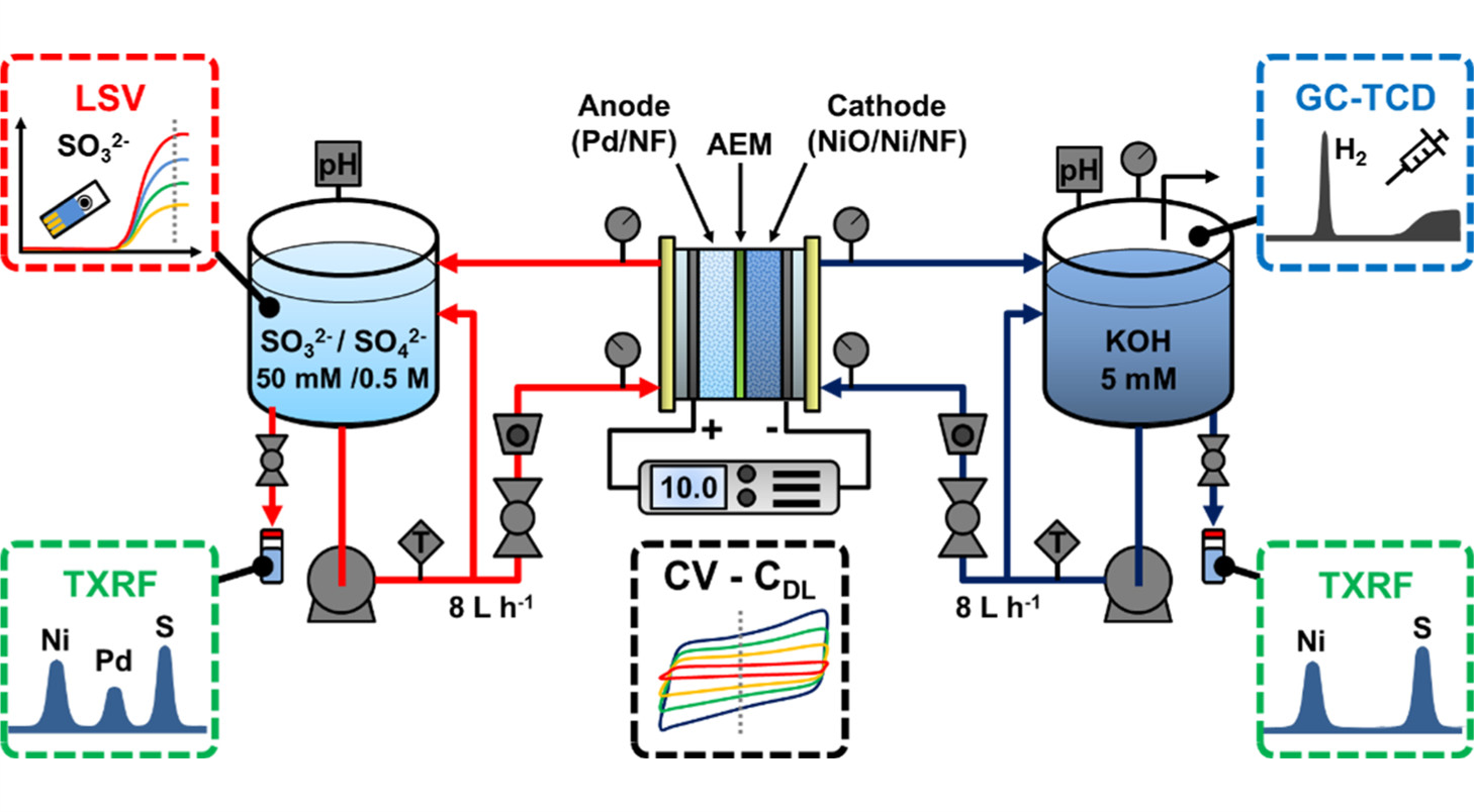
In a preliminary study, we demonstrated the viability of sulfite electrooxidation in a membrane reactor where sulfite ions are oxidized at the anode and high-purity hydrogen is generated at the cathode. In a subsequent study, our team at UACH then designed and optimized an electrochemical flow reactor for this process. We utilized various characterization techniques to assess the electrochemical step, including total-reflectance X-ray fluorescence (TXRF) for measuring catalyst dissolution, gas chromatography (GC) for hydrogen production rates, and voltammetric methods for sulfite detection (Figure 2).
We enhanced the catalytic activity and stability by depositing Pd films on Ni foam (Pd/NF) electrodes to maximize surface area. Using a custom-designed flow electrolyzer, we tested the performance of sulfite electrolysis and monitored sulfite oxidation and hydrogen production rates during batch electrolysis (Figure 3).